Image Galleries
Featured Article
 Electron Multiplying Charge-Coupled Devices (EMCCDs)
Electron Multiplying Charge-Coupled Devices (EMCCDs)
By incorporating on-chip multiplication gain, the electron multiplying CCD achieves, in an all solid-state sensor, the single-photon detection sensitivity typical of intensified or electron-bombarded CCDs at much lower cost and without compromising the quantum efficiency and resolution characteristics of the conventional CCD structure.
Product Information
Review Articles
Concepts in Digital Imaging Technology
Proximity-Focused Image Intensifiers
Image intensifiers were developed for military use to enhance our night vision and are often referred to as wafer tubes or proximity-focused intensifiers. They have a flat photocathode separated by a small gap on the input side of a micro-channel plate (MCP) electron multiplier and a phosphorescent output screen on the reverse side of the MCP.
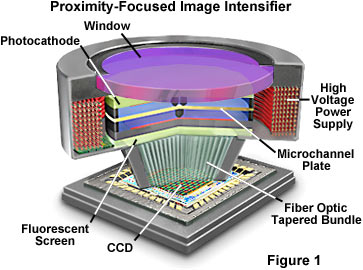
Substantial voltages are present across the small gaps between the photocathode, the phosphorescent output screen, and the MCP, which require careful construction of the devices to ensure they are free from contamination and can maintain high internal vacuums. Proximity-focused intensifiers are free from geometrical distortion or shading because the photoelectrons follow short, direct paths between the cathode, output screen, and the MCP rather than being focused by electrodes. Input and output windows are typically around 18 millimeters in diameter and consist of either a multialkali or bialkali photocathode (Gen II intensifiers) or a gallium arsenide photocathode (Gen III and Gen IV devices) and a P20 output phosphor. The overall photon gain of these devices averages about 10,000, which is calculated according to the equation:
Gain = QE x G(mcp) x V(p) x E(p)
where QE is the photocathode quantum efficiency (0.1 to 0.5 electrons/photon), G(mcp) is the microchannel plate gain (averaging between 500-1000), V(p) is the voltage between the MCP and the output phosphor (around 2500-5000 volts), and E(p) is the electron-to-light conversion efficiency of the phosphor (0.08-0.2 photons/electron). When the voltage drop between the MCP and the output phosphor decreases below 2500 volts, the phosphor becomes unresponsive.
The photocathode in the latest generation of these devices, while similar to that in photomultiplier tubes, has a higher quantum efficiency (up to 50 percent) in the blue-green end of the spectrum. The gain of the micro-channel plate is adjustable over a wide range with a typical maximum of about 80,000 (a detected photon at the input leads to a pulse of 80,000 photons from the phosphor screen). The phosphor matches the spectral sensitivity of the eye and is often not ideal for a CCD. Resolution of an intensified CCD depends on both the intensifier and the CCD, but is usually limited by the intensifier microchannel plate geometry to about 75 percent of that of the CCD alone. The latest generation of image intensifiers (denoted blue-plus Gen III or sometimes Gen IV; Figure 2) employ smaller microchannels (6 micron diameter) and better packing geometry than in previous models with a resultant substantial increase in resolution and elimination of the chicken-wire fixed-pattern noise that plagued earlier devices. The broad spectral sensitivity and high quantum efficiency (Figure 2) of the "high blue" GaAs and gallium arsenide phosphide (GaAsP) photocathodes are ideally suited to applications in fluorescence or low-light-level microscopy.
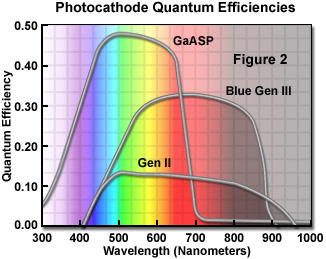
Image intensifiers have a reduced intrascene dynamic range compared to a slow-scan CCD camera and it is difficult to obtain more than a 256-fold intensity range (8 bits) from an intensified CCD camera. Intensifier gain may be rapidly and reproducibly changed to accommodate variations in scene brightness, thereby increasing the interscene dynamic range. Indeed, since image intensifiers can be rapidly gated (turned off or on in a few nanoseconds), relatively bright objects can be visualized by a reduction in the "on" time. A gated, variable gain intensified CCD camera is commercially available with a 12 order of magnitude dynamic range. Gated, intensified CCD cameras are required for most time-resolved fluorescence microscopy applications because the detector must be turned on and off in nanoseconds or its gain rapidly modulated in synchrony with the light source.
Thermal noise from the photocathode as well as electron multiplication noise from the microchannel plate reduce the signal-to-noise ratio in an intensified CCD camera to below that of a slow-scan CCD. The contribution of these components to the noise created by the statistical nature of the photon flux depends on the gain of the device and the temperature of the photocathode. Generally, a reduction of the gain of the intensification stage is employed to limit the noise although intensified CCD cameras are available with a cooled photocathode.
Intensified CCD cameras have a very fast response limited by the time constant of the output phosphor and often the CCD camera read out is the slowest step in image acquisition. Because of the low light fluxes emanating from the fluorochromes bound to or within living cells, intensified CCD cameras are frequently employed to study dynamic events and for ratio imaging of ion-sensitive fluorochromes. The simultaneous or near-simultaneous acquisition of two images at different excitation or emission wavelengths is required for ratio imaging and intensified CCD cameras have the requisite speed and sensitivity.
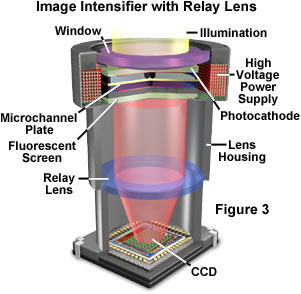
Two of the most popular approaches for relaying the output of an image intensifier to a video-rate camera (vidicon or CCD) are using an optical relay lens coupling or a fiber-optic coupling. Relay lenses are designed to capture light from the intensifier output window with minimal geometrical distortion or spherical aberration and project as much of the image as possible onto the video pickup device. The efficiency of a relay lens is given by the equation:
Efficiency = T/[4f2(1 + M2)]
where T is the lens transmission (around 0.9), M is the magnification (ranging between 0.5x and 2x), and f is the lens f-number (1.0 to 2.8). An ideal 1:1 relay lens with 100 percent transmission and an f-number of 1.0 will give a maximum transfer efficiency of only around 12 percent. When the input window of the video sensor (CCD array size) is smaller than the intensifier output window, the relay lens is required to demagnify the image to match the format of the sensor. Coupling efficiency increases proportionally with demagnification according to the efficiency equation given above. If the intensifier has sufficient gain and output luminance, the losses in the relay lenses may not adversely affect overall performance. Optical relay lenses work well with Gen II inverter tubes and some Gen III (or Gen IV) tubes coupled to Newvicon tube or CCD detectors because the high gain and high screen luminance of these intensifiers help to offset the inefficiency of the relay lenses.
The optimum method for coupling Proximity-focused image intensifiers to CCD sensors is through a fiber-optic taper (Figure 1). This approach achieves a coupling efficiency between 40 and 80 percent with matching formats, but requires a high degree of skill in bonding the fiber-optic taper to both devices. Maximum efficiency and minimal fixed-pattern noise are achieved when the CCD front window is removed and the fiber-optic taper is machined to fit directly onto the diode array surface. High resolution, artifact-free images require precision quality tapers having a small fiber diameter (between 2 and 3 microns) with very few missing or broken fibers and low fixed-pattern noise.
Use of optical relay lenses allows for convenient interchange of the video camera, CCD, and/or intensifier tube, and provides electrical isolation of the sensitive video camera input from the high voltages and high-frequency electrical interference present on the output of the image intensifier. Bonding fiber-optic tapers to the CCD surface is relatively permanent, and CCD failure can lead to loss of an expensive image intensifier and fiber-optic taper. To alleviate this problem, improvements in nonpermanent, optically matched, silicon bonding materials make it possible to disassemble fiber-optic coupled systems without destruction.
Contributing Authors
Kenneth R. Spring - Scientific Consultant, Lusby, Maryland, 20657.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.