Image Galleries
Featured Article
 Electron Multiplying Charge-Coupled Devices (EMCCDs)
Electron Multiplying Charge-Coupled Devices (EMCCDs)
By incorporating on-chip multiplication gain, the electron multiplying CCD achieves, in an all solid-state sensor, the single-photon detection sensitivity typical of intensified or electron-bombarded CCDs at much lower cost and without compromising the quantum efficiency and resolution characteristics of the conventional CCD structure.
Product Information
Digital Image Gallery
Fluorescence Microscopy Digital Image Gallery
Bovine Pulmonary Artery Endothelial Cells (BPAE Line)
The BPAE cell line was initiated in January 1979 by P. Del Vecchio from the main stem of a pulmonary artery belonging to a young cow (Bos taurus). Pulmonary arteries, which extend from the heart to the lungs, are the only arteries in the mammalian body that carry dark, unoxygenated blood.
The BPAE line of endothelial cells is positive for bovine diarrhea virus, one of the most important known bovine viral pathogens, which causes a broad array of clinical syndromes that result in significant losses in the beef industry each year. BPAE cells are also positive for angiotensin converting enzyme (ACE), an enzyme that is intricately involved in the maintenance of blood pressure and volume. Due to this fact, BPAE cells are often utilized in hypertension research as well as studies of atherosclerosis and coronary heart disease.
Endothelial cells, such as those of the BPAE line, are generally flat and elongated in shape. When found in the body lining the pulmonary arteries, these cells are usually arranged so that their long axis is oriented parallel to the direction of blood flow in the vessel. The cells are connected to one another via both gap junctions and tight junctions. An organized array of membrane channels, a gap junction allows adjacent cells to communicate through direct passage of small molecules and ions. In contrast, tight junctions act as diffusion barriers that restrict the dispersal of molecules to adjacent cells with varying degrees of success. Tight junctions are found at the most apical point between neighboring endothelial cells and form a band around the entire circumference of the cell.
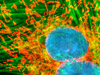
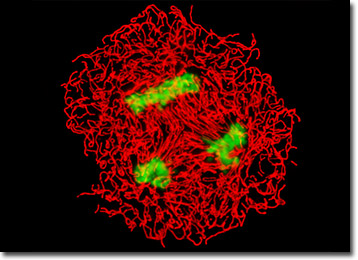
The adherent culture of BPAE cells featured in the digital image above was labeled with MitoTracker Red CMXRos and counterstained with SYTOX Green. Note the triplet of mitotic spindles present in the image, which are surrounded by a field of red mitochondria. Images were recorded in grayscale with a Hamamatsu ORCA-AG camera system coupled to a Nikon 80i upright microscope equipped with bandpass emission fluorescence filter optical blocks provided by Semrock. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Bovine Pulmonary Artery (BPAE) Cells
Examining nuclear pores, mitochondria, and filamentous actin in BPAE Cells - The adherent culture of BPAE cells featured in this section was labeled with MitoTracker Red CMXRos (mitochondria; pseudocolored yellow), Alexa Fluor 488 (filamentous actin; green), Alexa Fluor 647 (nuclear pore complex protein; red), and Hoechst 33342 (nucleus; cyan).
Immunofluorescence labeling of tubulin in BPAE Cells - The BPAE cells presented in this section were immunofluorescently labeled with mouse anti-alpha-tubulin primary antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 568. In addition, the specimen was stained with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33258, targeting the filamentous actin network and nuclei, respectively.
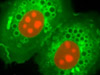
The Golgi Complex in BPAE Cells - A culture of BPAE cells was fixed, permeabilized, and blocked with 10-percent normal goat serum in phosphate-buffered saline prior to immunofluorescent labeling with rabbit primary antibodies to giantin, a protein resident in the Golgi complex of mammalian cells. The culture was subsequently stained with a mixture of goat anti-rabbit secondary antibody fragments (heavy and light chain) conjugated to Alexa Fluor 488. In addition, the cells were labeled with Alexa Fluor 568 conjugated to phalloidin (targeting the filamentous actin network), and for DNA with Hoechst 33342.
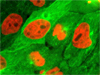
Mitochondria, Filamentous Actin, and Nuclear Staining in BPAE Cells - The BPAE cells illustrated in this section were labeled with MitoTracker Red CMXRos before fixing, and the cells were subsequently stained with Alexa Fluor 488 conjugated to phalloidin, followed by Hoechst 33258, a popular DNA-binding counterstain. Images were combined after applying digital pseudocolors corresponding with fluorophore emission profiles.
Four-Color Fluorescence with BPAE Cells - The bovine pulmonary artery endothelial (BPAE) cells presented in this section were resident in an adherent culture stained for F-actin with Alexa Fluor 488 conjugated to phalloidin (green fluorescence), and for DNA with the bis-benzimidazole dye Hoechst 33258 (blue fluorescence). In addition, the culture was immunofluorescently labeled with Alexa Fluor 568 conjugated to goat secondary antibodies that target mouse anti-PDI (protein disulfide isomerase) primary antibodies (red fluorescence). The mitochondrial network was simultaneously visualized using MitoTracker Deep Red 633 (pseudocolored yellow).
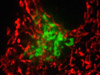
Highlighting Nuclear Pore Complex Protein in Endothelial Cells - Endothelial cells resident in a monolayer culture were immunofluorescently labeled with Alexa Fluor 488 conjugated to goat secondary antibodies that target mouse anti-NPCP (nuclear pore complex protein; green fluorescence). The cells were also counterstained with MitoTracker Red CMXRos (mitochondria; red fluorescence) and Alexa Fluor 350 conjugated to phalloidin (filamentous actin; blue).
Bovine Pulmonary Artery Endothelial Cells with Texas Red-X, Oregon Green 488, and Hoechst 33258 - In order to label the intermediate filaments in a log phase adherent BPAE culture, the fixed and permeabilized cells were blocked and treated with mouse anti-vimentin (porcine eye lens) primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Texas Red-X. Filamentous actin was visualized with phalloidin conjugated to Oregon Green 488, while the nuclei were stained with Hoechst 33258.