Image Galleries
Featured Article
 Electron Multiplying Charge-Coupled Devices (EMCCDs)
Electron Multiplying Charge-Coupled Devices (EMCCDs)
By incorporating on-chip multiplication gain, the electron multiplying CCD achieves, in an all solid-state sensor, the single-photon detection sensitivity typical of intensified or electron-bombarded CCDs at much lower cost and without compromising the quantum efficiency and resolution characteristics of the conventional CCD structure.
Product Information
Digital Image Gallery
Fluorescence Microscopy Digital Image Gallery
Tahr Ovary Epithelial Cells (HJ1.Ov Line)
The HJ1.Ov cell line was derived from the ovary tissue of a normal and healthy female Himalayan tahr (Hemitragus jemlahicus), which is a relative of the wild goat specially adapted to life in the rugged, mountainous environment of the Himalayas. Developed at The Naval Biosciences Laboratory (NBL) in Oakland, California, continuous cultures of HJ1.Ov cells exhibit typical epithelial morphology and grow adherently to glass and polymer surfaces in monolayer culture.
Epithelial cells comprise the avascular tissues that line both the interior and exterior surfaces of the body and its organs. Also, the secretory portions of glands and their ducts are formed from invaginated epithelial cells. Though there are many different types of epithelial cells in the body that may be arranged in a number of ways, the cells are always contiguous with one another so that they create an uninterrupted barrier.
The epithelium that surrounds the ovaries is continuous with the peritoneum that lines the body cavity. The modified tissue is generally known as the germinal epithelium, but the name is deceptive. The term was coined at a time when it was supposed that the simple cuboidal epithelium was the source of germ cells. It has since been realized that, in most adults, the tissue does not contain any germ cells, but the name persists. The germinal epithelium is capable of cell division, a process that is especially important for animals that experience significant expansion of the ovaries seasonally for breeding purposes. Located beneath this layer of tissue is the tunica albuginea, a relatively thin stratum of connective tissue that consists of parallel arrays of collagen fibers.
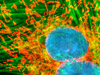
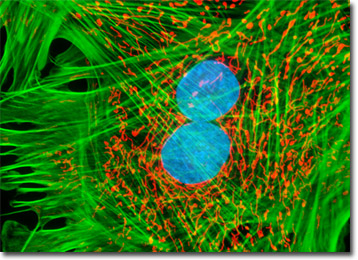
The adherent monolayer HJ1.Ov cell culture presented in the digital image above was labeled for the cytoskeletal filamentous actin and intracellular mitochondrial networks with Alexa Fluor 488 conjugated to phalloidin and MitoTracker Red CMXRos, respectively. Nuclei present in the epithelial cells were counterstained with the DNA-selective bisbenzimide dye, Hoechst 33258. Images were recorded in grayscale with a Hamamatsu ORCA-AG camera system coupled to an Olympus BX-51 microscope equipped with bandpass emission fluorescence filter optical blocks provided by Omega Optical. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Fluorescence Images of Tahr Ovary Epithelial (HJ1.Ov) Cells
Alexa Fluor Probes with UV, Blue, and Green Excitation in Tahr Cells - Employing a combination of ultraviolet, blue, and green excitation Alexa Fluor (350, 488, and 568, respectively) dyes, a monolayer culture of HJ1.Ov cells was triple-labeled using double immunofluorescence and a phallotoxin. Nuclei were visualized with mouse anti-NPCP (nuclear pore complex protein) primary antibodies, while the Golgi complex was stained with rabbit anti-giantin antibodies.
Examining the Cytoskeletal Networks in Tahr Ovary Cells - An adherent culture of tahr ovary epithelial cells was treated with mouse anti-vimentin primary antibodies followed by goat anti-mouse secondary antibody Fab fragments conjugated to Cy3. The culture was also counterstained with Alexa Fluor 488 conjugated to phalloidin (to highlight filamentous actin) and Hoechst 33342.
Four-Color Fluorescence Imaging with Epithelial Cells - In a double immunofluorescence labeling protocol, adherent tahr ovary cells were fixed, permeabilized, and treated with a cocktail of mouse-anti-vimentin and rabbit anti-giantin primary antibodies, targeting the intermediate filaments and Golgi complex, respectively. The culture was counterstained with Alexa Fluor 488 conjugated to phalloidin and TO-PRO-3 to provide four colors.
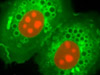
Targeting the Nuclear Pore Complex Proteins, Filamentous Actin Network, and Nuclear DNA in HJ1.Ov Cells - A culture of normal tahr ovary cells was fixed, permeabilized, blocked with 10-percent normal goat serum, and treated with mouse anti-NPCP (nuclear pore complex protein) primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Alexa Fluor 568 (red fluorescence). Subsequently, the filamentous actin network and nuclear DNA were labeled with Alexa Fluor 488 conjugated to phalloidin and Hoechst 33258, respectively.
The Mitochondrial Network in Tahr Ovary Cells - An adherent monolayer culture of normal tahr ovary epithelial cells was immunofluorescently labeled with primary mouse anti-oxphos complex V inhibitor protein antibodies, followed by goat anti-mouse Fab fragments conjugated to fluorescein. The culture was subsequently stained with Alexa Fluor 568 conjugated to phalloidin to reveal details of the filamentous actin network, and DAPI for DNA in the nucleus.
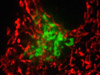
Visualizing Peroxisomes and Filamentous Actin in Tahr Ovary Cells - In a double immunofluorescence experiment, fixed and permeabilized adherent HJ1.Ov cells were treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by a second mixture of goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively (targeting the nucleus and peroxisomes). The filamentous actin cytoskeletal network was stained with Alexa Fluor 350 conjugated to phalloidin.
Examining Mitochondria Proximity to the Nucleus in Epithelial Cells - Live tahr ovary cells were treated with MitoTracker Red CMXRos in media containing calf serum for one hour followed by fixation with paraformaldehyde. The fixed cells were then counterstaned with Hoechst 33258 to highlight nuclei in order to examine the spatial relationship between mitochondria and the nucleus in this cell line.
Intermediate Filaments and the Cytoskeleton in Tahr Ovary Cells - A log-phase culture of HJ1.Ov cells was fixed, permeabilized, blocked with 10-percent normal goat serum and treated with mouse anti-vimentin primary antibodies followed by goat anti-mouse secondary Fab fragments conjugated to Alexa Fluor 568. The cells were counterstained with phalloidin conjugated to Alexa Fluor 633 and SYTOX Green.
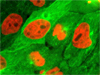
Distribution of Keratin and Mitochondria in HJ1.Ov Cell Cultures - An adherent log phase culture of tahr ovary cells was treated for one hour with MitoTracker Red CMXRos in order to label the mitochondrial network, and the fixed cells were then incubated with mouse anti-cytokeratin primary antibodies followed by goat anti-mouse secondary antibodies (IgG) conjugated to Alexa Fluor 488. The nuclei were counterstained with Hoechst 33258.
Proximity of the Golgi Complex and Nucleus in Normal Tahr Ovary Monolayer Cell Cultures - The close proximity between the Golgi complex and nuclei in HJ1.Ov cells was probed in a double immunofluorescence experiment with mouse anti-NPCP (nuclear pore complex protein) and rabbit anti-giantin primary antibodies. The antibody targets were visualized with goat secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively, while the actin cytoskeletal framework was labeled with Alexa Fluor 350 conjugated to phalloidin.
Localizing Actin, Mitochondria, and the Nucleus in HJ1.Ov Cells - By applying the popular triple-fluorophore combination of MitoTracker Red CMXRos, Alexa Fluor 488 conjugated to phalloidin, and Hoechst 33258 to a culture of normal tahr ovary cells, the filamentous actin (green) and mitochondrial (red) networks are revealed, as is the location of the nucleus (blue).